First Law of Thermodynamics
First Law of Thermodynamics: Overview
This Topic covers sub-topics such as First Law of Thermodynamics, Sign Convention in Thermodynamics, First Law of Thermodynamics in Isothermal Process, First Law of Thermodynamics in Adiabatic Process and, Heat in Isothermal Process
Important Questions on First Law of Thermodynamics
Given below are two statements:
Statement I: If heat is added to a system, its temperature must increase.
Statement II: If positive work is done by a system in a thermodynamic process, its volume must increase.
In the light of the above statements, choose the correct answer from the options given below
A source supplies heat to a system at the rate of If the system performs work at a rate of The rate at which internal energy of the system increases is
One mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in fig. Calculate
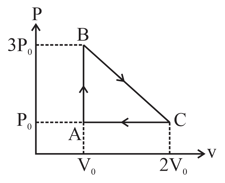
(a) The work done by the gas.
b) The heat rejected by the gas in the path and heat absorbed in the path .
(c) The net heat absorbed by the gas in the path .
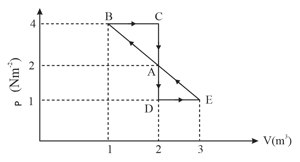
One mole of monoatomic gas is carried along process as shown in the diagram. Find the net work done by gas.
The amount of heat supplied to a gas in a system is equal to , the system in return does of work on the surrounding. Find change in internal energy of the gas.
If amount of heat given to the system be Joules and the amount of work done by the system is . If initial internal energy of the system is . Final internal energy of the system is
ideal gas expands from volume to,. This may be achieved by either of the three processes: isobaric, isothermal and adiabatic. Let be the change in internal energy of the gas, be the quantity of heat added to the system and W be the work done by the gas. Identify which of the following statements is false for?
of water at atmospheric pressure has volume of and when boiled it becomes of steam. The heat of vaporization of water is . Then the change in its internal energy in this process is
How the first law of thermodynamics can be justified? Give an example in support of your answer.
An ideal gas is expanded adiabatically at an initial temperature of so that its volume is doubled. The final temperature of the hydrogen gas is
In an insulated container of water is stirred with a rod to increase the temperature. Which of the following is true?
of water is heated from to , if its volume remains constant, then the change in internal energy is (specific heat of water )
of liquid water at undergoes a phase change into steam at at atm (take it to be ). The initial volume of the liquid water was which is changed to of steam. Find the change in the internal energy of the system.
[Use heat of vaporization ]
State first law of thermodynamics.
Consider the following cycle (a-b-c) for ideal gas in the figure: b-c(adiabatic), c-a(isobaric), and a-b (isochoric). Calculate work done by the gas and heat absorbed by the gas for each path, a-b, b-c, c-a. What is the total work done and heat absorbed for the full cycle?
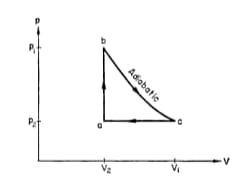
In the diagram shown and cal. If cal for the curved path value of for path , will be
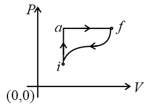
Which of the following is the correct equation?
A cube of side made of iron and having a mass of is heated from to . The specific heat for iron is and the coefficient of volume expansion is , the change in the internal energy of the cube is (atm pressure )
If of heat is supplied to gas at a pressure of , its volume increases from to . Calculate the change in its internal energy.
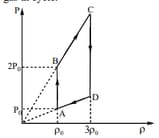
moles of an ideal gas is taken through a cyclic process . Pressure - density diagram of which is shown in adjacent figure. If molar mass of gas is , what will be the work done by gas in cycle.